What Does It Mean When a Reaction Is Spontaneous Apex
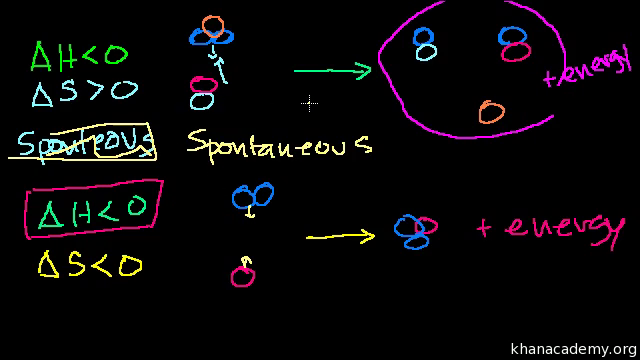
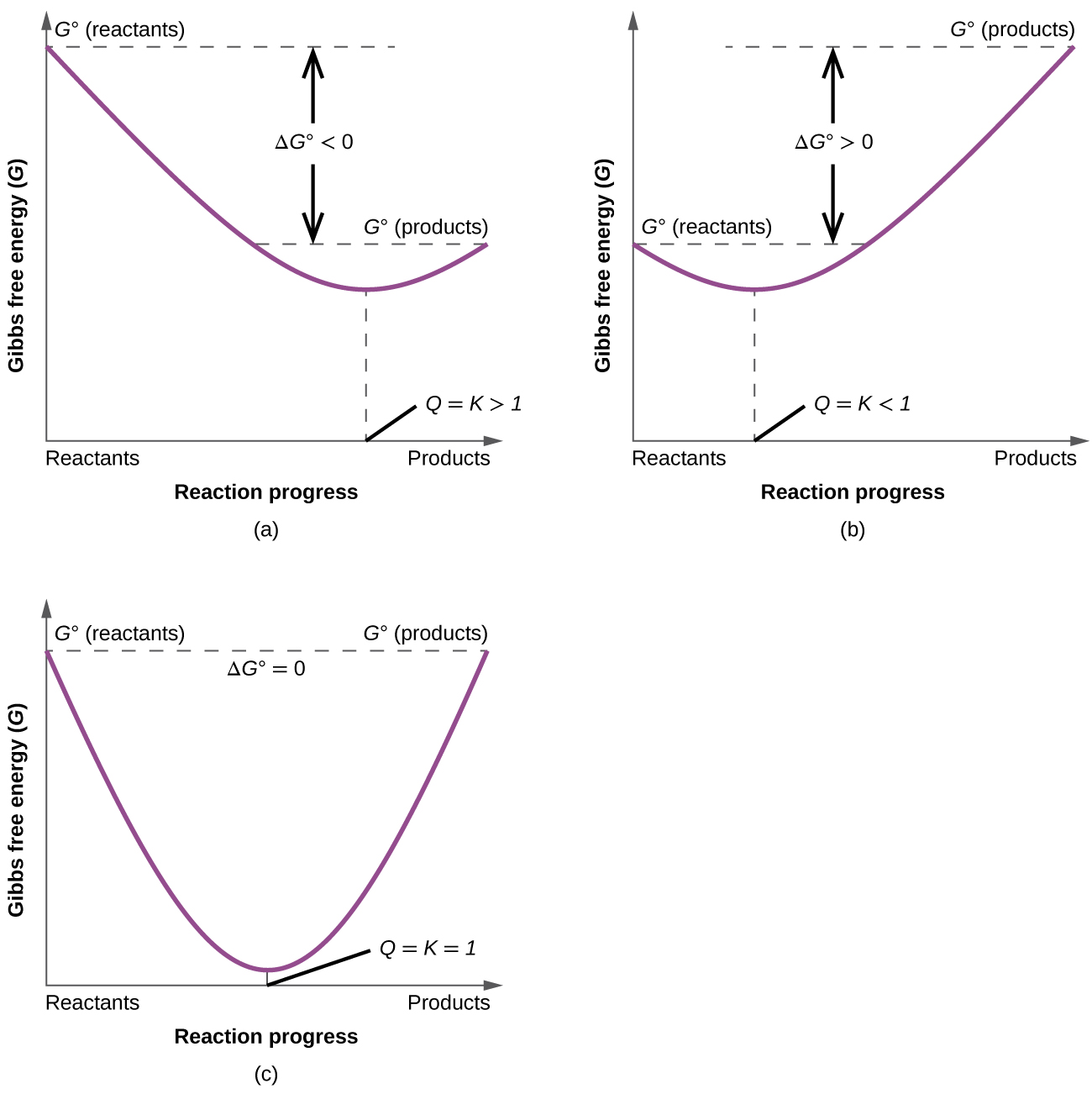
A spontaneous reaction is a reaction that occurs in a given set of conditions without intervention. A spontaneous reaction may involve an increase or decrease in enthalpy it may involve an increase or decrease in entropy but it will always involve a decrease in free energy that is a negative ΔG.
According to hesss law the overall enthalpy of a reaction is the difference between the sum of enthalpy values of all the products and the sum of enthalpy values of all the reactants.

. A roaring bonfire see figure below is an example of a spontaneous reaction. The term spontaneous is not a synonymous of possible. What is the value for G at 500 K if H 27 kJmol and S 009 kJmolK.
23 Can a spontaneous process be undone. A roaring bonfire is an example of a spontaneous reaction since it is exothermic there is a decrease in the energy of the system as energy is released to the surroundings as heat. 88 kj or 88 kj.
There is no force preventing these atoms from reacting together. A spontaneous chemical reaction or physical change is one that once started will continue without any outside help. What does a positive cell voltage mean.
You will need to refer to a table of Standard Reduction Potentials in order to determine the standard electrode potential for a. Start studying 524 Quiz. A spontaneous reaction is a reaction that favors the formation of products at the conditions under which the reaction is occurring.
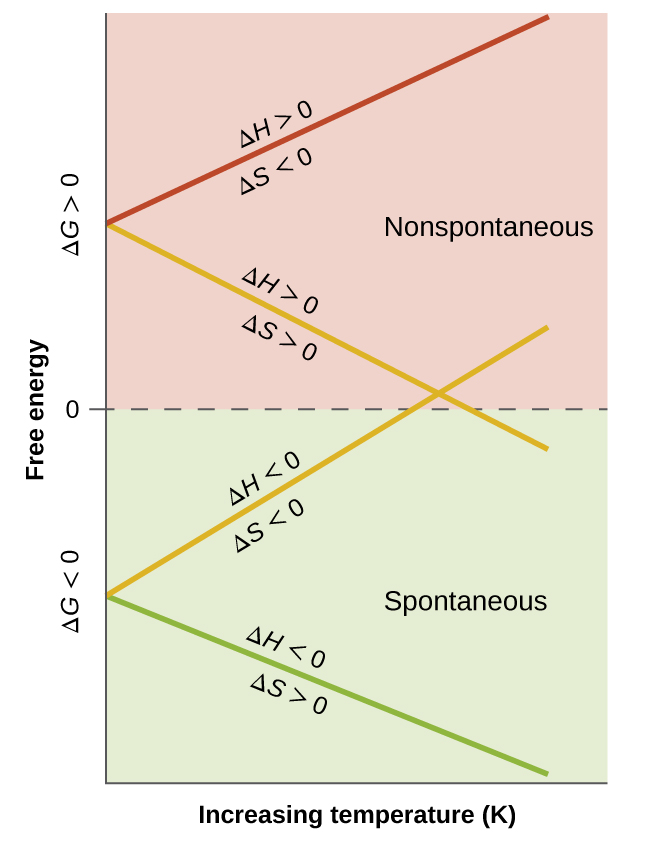
So clearly a spontaneous reaction is possible with both when entropy change is negative and also when entropy change is positive. 24 Is a spontaneous reaction always fast. The entropy change ΔS of the reaction is negative.
Under what conditions will a low temperature make a reaction spontaneous. What does a negative change in entropy indicate. That is there is no force acting against them.
G -18 kJmol. It means that except for the bodies taking part in the process there are no permanent changes of any sort and there is no need of input work. It is rather a synonymous of natural.
This is a reaction that takes place on its own without an external force and another reaction needed to drive it. So entropy change is positive. 22 What is an example of a non-spontaneous reaction.
Occurring without added energy. The meaning of spontaneous processreaction in thermodynamics is quite straightforward. A spontaneous reaction is a reaction that favors the formation of products at the conditions under which the reaction is occurring.
For example the reaction between calcium and carbon dioxide is spontaneous. Learn vocabulary terms and more with flashcards games and other study tools. If E o for the redox reaction as written is negative the reaction DOES NOT proceed in the forward direction and is said to be non-spontaneous.
It might not take. It is a spontaneous reaction. A fire is exothermic which means a decrease in the energy of the system as energy is released to the surroundings as heat.
21 What does it mean when a reaction is spontaneous apex. What does the term spontaneous mean in chemical reactions. Enthalpy and Entropy Chemistry Tutorial Key Concepts.
The enthalpy of the overall chemical reaction is 87. If H and S are both negative. Spontaneous reactions are accompanied by an.
25 What is a spontaneous exothermic reaction. However some reactions are also inhibited by other substances. A nonspontaneous chemical reaction or physical change is.
Reactions are always preferred when they are spontaneous.

Enzymes Effect On Activation Energy And Free Energy Youtube
Gibbs Free Energy And Spontaneity Video Khan Academy

What Causes A Reaction To Be Spontaneous Lisbdnet Com

Pin By Elizabeth Rivers On Dental Endodontics Dental Discomfort

Gibbs Free Energy And Spontaneity Article Khan Academy

Gibbs Free Energy And Spontaneity Article Khan Academy

What Does It Mean When A Reaction Is Spontaneous Lisbdnet Com

What Causes A Reaction To Be Spontaneous Lisbdnet Com

Gibbs Free Energy And Spontaneity Article Khan Academy

What Does It Mean When A Reaction Is Spontaneous Lisbdnet Com
13 7 The Gibbs Free Energy Chemistry Libretexts

Above What Temperature Does The Following Reaction Become Nonspontaneous Lisbdnet Com

Above What Temperature Does The Following Reaction Become Nonspontaneous Lisbdnet Com

Gibbs Free Energy And Spontaneity Video Khan Academy
How Is Enthalpy Related To The Spontaneity Of A Reaction Quora
13 7 The Gibbs Free Energy Chemistry Libretexts

What Causes A Reaction To Be Spontaneous Lisbdnet Com

What Does It Mean When A Reaction Is Spontaneous Brainly Com
Comments
Post a Comment